Data from small cohorts of patients (pts) with chronic lymphocytic leukemia (CLL) relapsing after venetoclax-based fixed-duration regimens suggest that venetoclax retreatment may be a feasible option. Information on the efficacy and tolerability of venetoclax retreatment in this setting is still scanty, making it worth exploring venetoclax retreatment in prospective cohorts of well-characterized pts with CLL. We analyzed the efficacy and safety of venetoclax retreatment in pts with relapsed/refractory CLL enrolled in our clinical trial IMPROVE (NCT04754035).
BTK- and BCL2-inhibitor naïve pts enrolled into the study were treated with venetoclax single-agent for 12 months; those with undetectable measurable residual disease (uMRD) discontinued therapy while those with detectable MRD received the combination of ibrutinib and venetoclax until uMRD, toxicity or for up to 12 months. Pts with detectable MRD at the end the combination therapy stopped venetoclax and continued with ibrutinib only.
In pts who experienced progressive disease (PD) requiring treatment, venetoclax single-agent was restarted according to the standard schedule and continued until PD or unacceptable toxicity. MRD was tested every 3 months in peripheral blood using the 6-color flow cytometry panel validated by the ERIC (European Research Initiative on CLL).
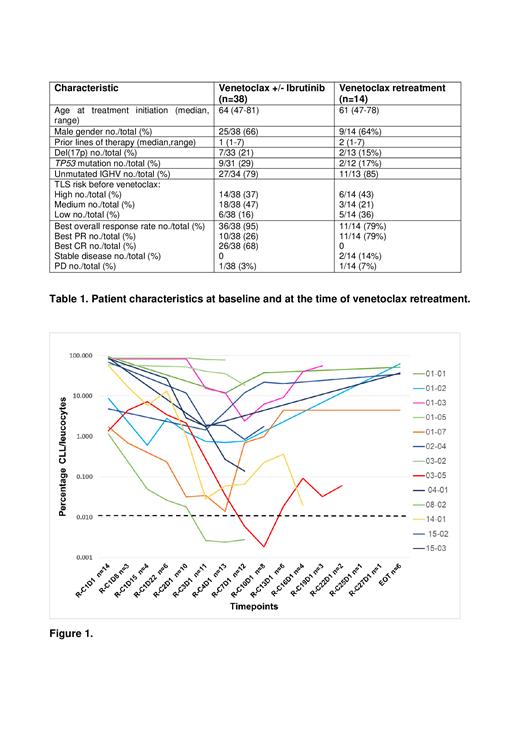
After a median follow-up of 58 months (5-67) from C1D1, 20/38 (52.6%) pts experienced PD requiring treatment after discontinuing therapy, including 19 upon reaching confirmed uMRD (11 treated with venetoclax single-agent, 8 with the combination of venetoclax+ibrutinib), 1 who discontinued both venetoclax and ibrutinib in partial response (PR) with detectable MRD (dMRD) due to an unrelated adverse event. Two out of 20 had Richter transformation (RT), 2 developed autoimmune hemolytic anemia, 2 received other treatments (BTK inhibitors) per investigator's decision and all 6 went off study. The remaining 14 pts (1 with detectable MRD, 7 who previously achieved uMRD with venetoclax monotherapy, 6 who were treated with the combination of venetoclax+ibrutinib) received venetoclax retreatment at a median of 32.5 months (12-44) after the end of initial therapy (Table 1); to note, the pt who stopped treatment in PR with dMRD restarted venetoclax after only 13 months. All 13 uMRD subjects experienced MRD relapse at a median of 5 months (0-41) after stopping treatment, 26 (0-37) months before disease progression requiring therapy. At the time of venetoclax initiation tumor lysis syndrome (TLS) risk was high in 6, medium in 3, low in 5. Best overall response rate was 79% (11/14), all partial responses (PR), 2 pts experienced stable disease (on treatment for 2 and 24 months).
With a median time on treatment of 10 months, 2 pts achieved uMRD after 3 and 6 months, the second one maintaining uMRD for 6 months and then becoming MRD positive again (Figure 1). Five out of 14 pts progressed after 2, 7, 9 and 11 (n=2) months on venetoclax retreatment, 3/5 received next-line treatment with ibrutinib, 2/5 received non-covalent BTK inhibitor and all achieved a PR. At last follow-up, 57% of pts remain on venetoclax; 12/14 patients undergoing venetoclax retreatment were alive (1 death due to RT on ibrutinib, 1 due to CLL progression on non-covalent BTK inhibitor). Progression-free survival from venetoclax restart to PD on venetoclax retreatment, last follow-up or death was not reached (0-28 months).
Venetoclax retreatment was very well tolerated with no patients experiencing TLS, 6/14 having grade 3-4 neutropenia (only in 1 case leading to treatment discontinuation), 8/14 infections (2 grade 1, 4 grade 2, 2 grade 3). No other grade 3/4 hematologic or non-hematologic toxicities were observed.
Venetoclax retreatment was effective and well-tolerated in a cohort of pts with relapsed/refractory CLL who have previously received time limited, MRD-guided treatment with venetoclax+/-ibrutinib. MRD relapse occurred at a median of about 2 years before clinical progression requiring treatment. Venetoclax retreatment was started at a median of 32.5 months after the end of initial therapy. Nearly 80% of patients achieved at least a partial response with venetoclax retreatment, 14% of pts achieved uMRD and 57% remain on venetoclax, with a median time on treatment of 10 months.
Disclosures
Albi:AstraZeneca: Honoraria. Scarfo:AbbVie: Consultancy; AstraZeneca: Consultancy; BeiGene: Consultancy; Janssen: Consultancy; Lilly: Consultancy; Octapharma: Speakers Bureau. Laurenti:AstraZeneca: Membership on an entity's Board of Directors or advisory committees; Beigene: Membership on an entity's Board of Directors or advisory committees; Janssen: Membership on an entity's Board of Directors or advisory committees; Abbvie: Membership on an entity's Board of Directors or advisory committees. Varettoni:ABBVIE: Honoraria, Membership on an entity's Board of Directors or advisory committees; ASTRAZENECA: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; BEIGENE: Honoraria, Membership on an entity's Board of Directors or advisory committees; JANSSEN: Honoraria, Membership on an entity's Board of Directors or advisory committees. Ghia:Lilly/Loxo Oncology: Consultancy, Honoraria, Research Funding; Janssen: Consultancy, Honoraria, Research Funding; AstraZeneca: Consultancy, Honoraria, Research Funding; MSD: Consultancy, Honoraria, Research Funding; Roche: Consultancy, Honoraria, Research Funding; BMS: Consultancy, Honoraria, Research Funding; BeiGene: Consultancy, Honoraria, Research Funding; AbbVie: Consultancy, Honoraria, Research Funding.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal